
Back to the Accelerated Bio web page.
The Food Lab page. *make food lab a hyperlink*
The Egg Lab

Welcome to Mr. Warren's class's egg lab page. We were learning about diffusion and osmosis, so our teacher decided to make a lab that will apply what we have learned. To start this off, observe the eggs in the bin of vinegar above.

If you don't already know, vinegar dissolves the shell of the egg, leaving a membrane permeable to water.

The eggs now have no shell. But that also means they can break easier....

Oh well.
The purpose of this lab was to see how osmosis works. First, we had to weigh the eggs to determine their initial mass.
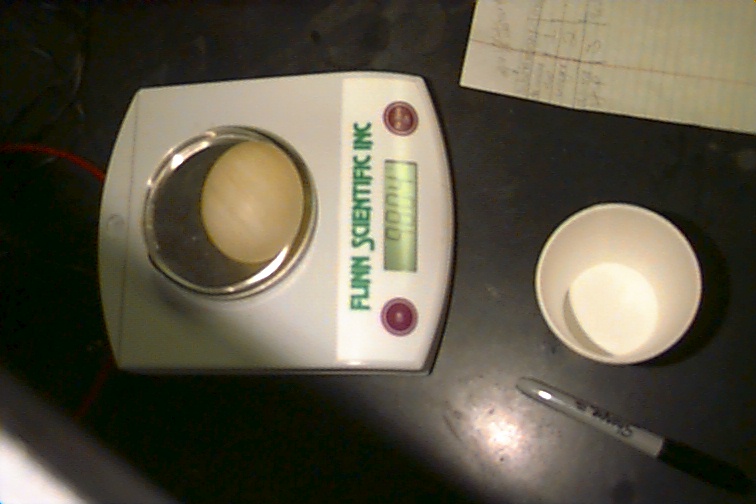
From there, we could determine how the egg's mass changed when we do the lab. Now, after the mass is recorded from the three eggs, we can put one in a cup of water, one in a cup of syrup, and one in a cup of vinegar. Then, they were set out over night.

The next day, the eggs were removed from their respective cups, dried, and weighed again. The new mass and change in mass were recorded. If the egg's new mass was more than it was before, that means some of the liquid surrounding the egg diffused/osmosed into it. The surrounding liquid was HYPERTONIC to the egg.
If the egg's new mass is less than before, that means water moved from the inside of the egg to the outside. The liquid surrounding the egg was HYPOTONIC.
Here's an example of what some of the results were.
| before mass | after mass | conclusion (outside to inside egg) | |
| syrup | 20.08g | 19.8g | hypertonic |
| vinegar | 23.4g | 23.4g | isotonic |
| water | 25.6g | 26.0g | hypotonic |
And remember, a good student cleans up after themselves!

Definitions:
HYPERTONIC: Higher concentration of water than,
HYPOTONIC: Lower concentration of water than,
ISOTONIC: Same concentration of water as,
Back to the Accelerated Bio web page.
Now onto the Food Lab... *make foodlab a hyperlink*
| Page by Chris Lamothe and Mike Buchannan. Last Updated February 6, 2004. |